
Results of the Inquiry on Bioethics
Results of the Inquiry on Bioethics
Bioethics Working Group WP6
Manuela M Bergmann 1,
Marek Bodzioch 2,
Luisa Bonet 3,
Catherine Defoort 4,
Georg Lietz 5
1 German Institute of Human Nutrition, Potsdam-Rehbrücke (Germany), Department. of Epidemiology
2 The Jagiellonian University (Poland), Medical College, Department of Clinical Biochemistry,
3 University of the Balearic Islands, Palma de Mallorca (Spain), Laboratory of Molecular Biology, Nutrition and Biotechnology
4 UMR INSERM (France), Faculté de médicine Timone
5 Newcastle University (UK), School of Clinical Medical Sciences, Human Nutrition
Major Purposes
To collect information on ...
- experiences of NuGO scientists with ethical approval for their studies
- opinions of the scientists about the 4 ethical issues
- the most sensitive conflicts between Ethics Committees and scientists
Additional Purposes
To collect information on ...
- existing and applied rules and regulations for ethical approval among NuGO member institutions
- laws and documents used by the Ethics Committees
- the affiliation and the address of the responsible Ethics Committees
Methods
Instruments
- Questionnaire which was online from October 7, 2004 - January 3, 2005
- electronic invitation to fill in the questionnaire
- reminder to those who did not answer:
- November 8, 2004
- November 29, 2004
- January 5, 2005
Study Subjects
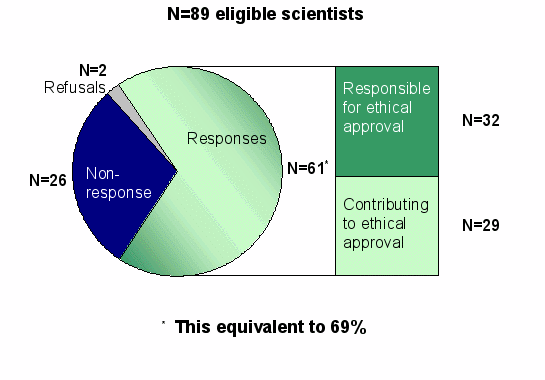
- 640 scientists had been registered in the NuGO network (Topshare) on October 1, 2004
- of which 551 were not involved in ethical approval or are not conducting human studies
- leaving 89 eligible scientists who could answer the questionnaire
Structure of the Questionnaire
- Introduction
- General questions
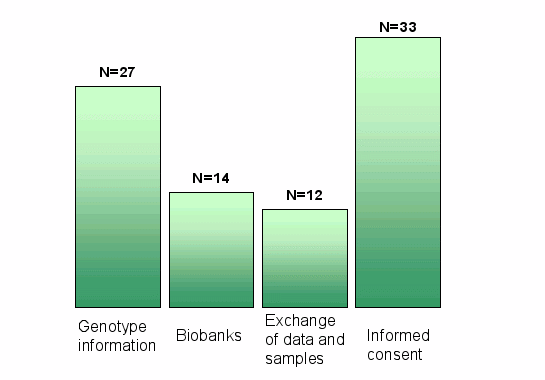
- Issue 1 to 4
- genotype information,
- biobanks,
- exchange of data and samples,
- informed consent)
- General questions
Online Questionnaire

Response
Response to Single Parts of the Questionnaire
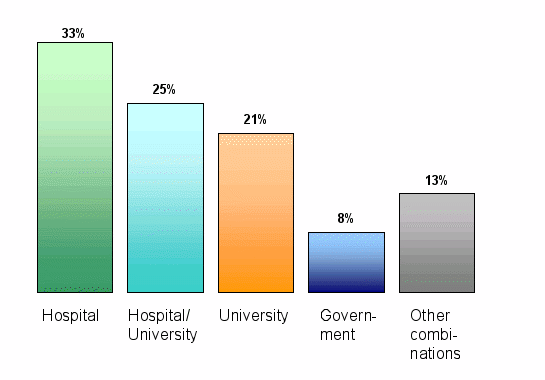
Affiliation of the Ethics Committee
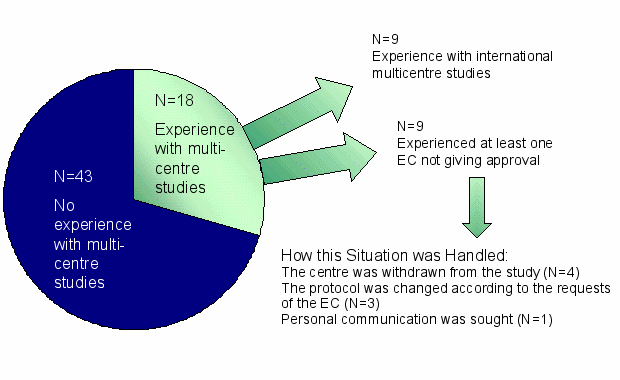
Ethical Approval in Multi-centre Studies
Key Points of the Open Answers
Genotype Information
- Position regarding disclosure of test results varies among EC and among scientists:
- No disclosure in any case to disclosure must be offered
- Crucial point, more frequently pointed to by the scientists: Health relevance of the information
- ECs and scientists think: If disclosed, information should only be given on a personal basis via qualified personnel
Biobanks
- The ECs' opinion about the propriety status of the sample vary:
- The sample is a donation (no one's property),
- The sample stays the property of the individual,
- The sample passes over into the possession of the researcher (institution)
- Most ECs stipulates the samples to be stored coded or anonymized
- However some ECs tolerate nominal storage
- There is confusion among the scientists about the meaning of
- Anonymous, anonymized, coded, or identified samples.
- Not mentioned at all: the quality management and control of biobanks
Exchange of Data and Samples
- Consensus exists between ECs and scientists: the confidentiality and protection of privacy must be guaranteed
- Exchange of data and samples is proved to be difficult when not subject matter in the initial informed consent
- The information or ex post consenting of the participants often is not feasible
- Scientists want not too strict regulations
Informed Consent
- Required accuracy and degree of detail with which planned tests have to be mentioned in the informed consent
- Potential field of conflicts between ECs and scientists
- Predefinition of the length of time of sample and data storage (by law, individually)
- Heterogeneous prerequisites for secondary use of samples
- Renewal of informed consent
- Anonymization
- EC's approval (amendment or renewal)
- Controversial rules about what to do with the sample (and data) when consent was withdrawn
- Can be kept and used/not used
- Must be destroyed immediately
- Can be kept until end of the study
Summary
Essential Issues for Researchers
- What laws regulate biobanking, exchange of samples and data and secondary use nationally and internationally
- How rigid are the procedures for extended storage of existing samples (biobanking) and secondary use
- How flexible is the informed consent regarding upcoming tests that have not been foreseen when the study was planned
- What is the optimal design of an informed consent (for future studies)
Essential Issues for the Individual
- Disclosure of genotype information and its consequences
- Quality of life, health consequences
- Legal consequences regarding obligation to inform insurances or employers
- To be able to give really free and really informed consent
- Comprehension of the participant and level of language presented by the researcher
- Motivation of participation in research
- Expected benefit due to participation
- If wished, to be able to keep track of what happens to the sample given
Conclusion
- We need...
- guidelines to follow for NuGO scientists and Ethics Committees
- education of scientists in these guidelines with the aim to improve the understanding of ethical implications (Seminars, e-learning, counseling for researchers seeking ethical approval etc.)
- to understand that these guidelines will be dynamic, meaning that they will have to be continuously adapted to new laws, regulations, research methods and findings







